Pink Sheet: Advisory Committee Disagreement With US FDA On Approval Decisions An Increasingly Rare Event

Pink Sheet published an article on our most recent study evaluating the alignment of FDA decisions with advisory committee recommendations and chatted with 3D’s co-founder, Jim DiBiasi, about the results. Read Derrick Gingery’s article below. This article was originally featured in Pink Sheet on January 24, 2023.
A NEW STUDY FINDS the agency only approved a product despite a negative advisory committee vote six times in six years. The overall rate of agreement between the agency and advisory committees has increased since 2015.
The shock and outrage in some circles over the US Food and Drug Administration’s surprise accelerated approval of Biogen, Inc.’s Alzheimer’s treatment Aduhelm (aducanumab-avwa) in 2021 stemmed partly from the agency’s disagreement with its outside advisers, an already uncommon event that data suggests is becoming even more rare.
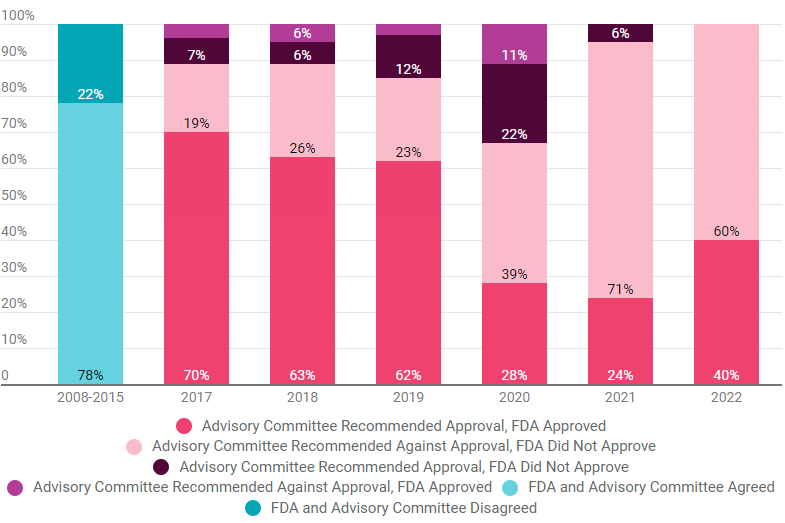
A study by 3D Communications of all FDA advisory committee votes and drug, biologic and device application decisions from 2017 through 2022 found that the FDA agreed with committee decisions 86% of the time. (See chart below.)
The data indicate an 8% increase in FDA-advisory committee agreement from a concordance study of agency decisions and advisory committee votes from 2008 through 2015. (Also see "US FDA, Advisory Committees Rarely Disagree" - Pink Sheet, 15 Jul, 2019.)
“Biogen was sort of an outlier,” said Jim DiBiasi, co-founder of 3D Communications, a firm that helps sponsors prepare for advisory committee meetings and other sessions, which conducted the new study. “It’s very, very rare [to see discordance].”
Meetings where the FDA had not yet ruled on the application, as well as Emergency Use Authorizations for COVID-19 products, were excluded from the study.
FDA officials convene advisory committees for many reasons, often to discuss pending applications. The agency is not required to follow an advisory committee recommendation.
DiBiasi said that the study found the high concordance rate even though it counted application rejections, i.e. complete response letters, conservatively. If a CR was the first action after the advisory committee meeting, it was considered a non-approval in the study, even if the product was approved later.
In five of the six years during the study period, the agreement rate was higher than the 78% rate found from 2008-2015. In 2022, the FDA agreed with all advisory committee recommendations, up from 94% in 2021, according to 3D Communications data.
In part because of its rarity, when the agency and committee do not agree, the uproar can be substantial. Three members quit the Peripheral and Central Nervous System Drugs Advisory Committee after Aduhelm was approved despite its virtually unanimous rejection of the product. (Also see "Aduhelm Approval Firestorm Raises Question: What Are US FDA Advisory Committees For, Anyway?" - Pink Sheet, 11 Jun, 2021.)
The story continues after the chart …
Adcomm, FDA Agreement Increased In Recent Years
A study of product decisions since 2017 showed agreement rates higher than a study of 2008 to 2015 rates, suggesting that misaligned decisions are becoming rarer.
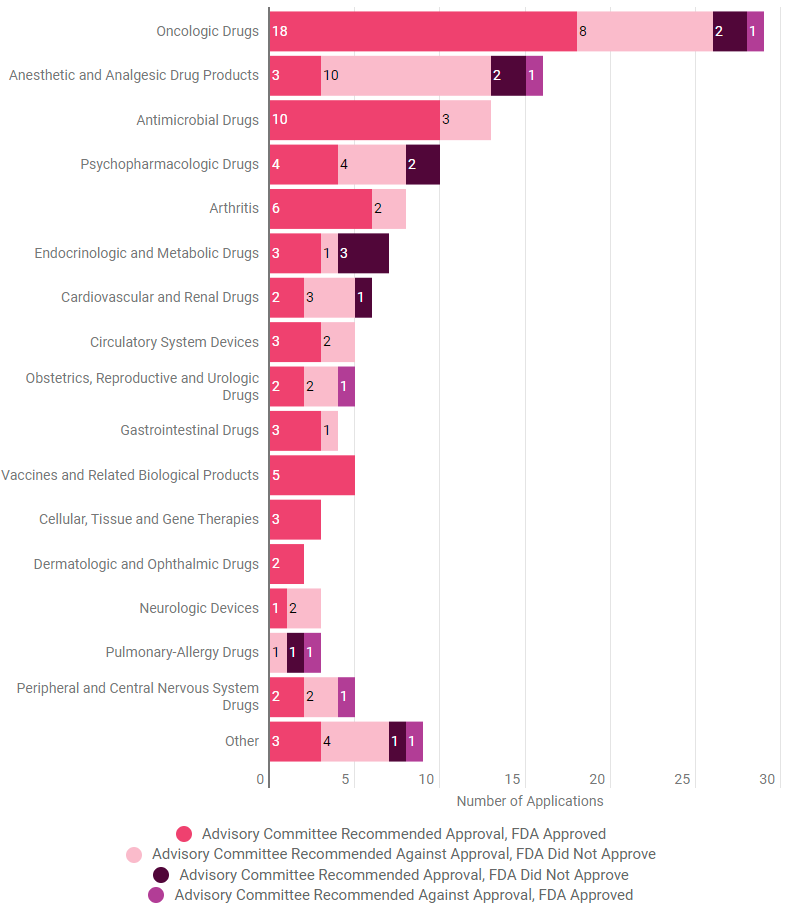
Some Committees More Agreeable With FDA Than Others
Could Rare Disagreements Prompt Process Changes?
The Aduhelm decision was the only time during the six-year period of the 3D Communications study that the FDA did not follow a PCNSDAC recommendation. The agency’s four other decisions during that time agreed with the committee finding (two approvals and two denials following the same recommendations).
The study found only six instances out of 133 meetings across all drug, biologic and device advisory committees during the period where the agency approved a drug the committee rejected. There were 12 instances where the FDA rejected a product that an advisory committee had recommended approval. Three were reviewed by the Endocrinologic and Metabolic Drugs Advisory Committee and two each by the Oncologic Drugs Advisory Committee, Anesthetic and Analgesic Drug Products Advisory Committee and Psychopharmacologic Drugs Advisory Committee.
Still, the uproar over Aduhelm seems to have prompted a review that could lead to advisory committee process changes.
“I think you may see a shift in FDA’s thinking about when to use advisory committees, even possibly how to staff advisory committees, how to frame questions for advisory committees,” said Geoffrey Levitt, of counsel at DLA Piper and co-chair of the firm’s FDA Regulatory Practice Group. “A much more speculative question is whether opinion leaders in certain fields will start rethinking their approach to serving on advisory committees. I haven't seen any evidence of this.”
FDA officials want discussions to focus on scientific issues and include less emotion. FDA Commissioner Robert Califf has said reform discussions could begin this year. (Also see "US FDA’s Califf Expects Advisory Committee Reform Talk ‘About A Year From Now’" - Pink Sheet, 29 Apr, 2022.)
The 3D Communications analysis also arrived on the heels of an investigation by two congressional committees that criticized the Aduhelm approval process and suggested several changes, including creating guidelines for using joint-FDA-sponsor briefing packages for advisory committee meetings.
Agency officials also told House investigators that they decided to clear Aduhelm using accelerated approval, despite telling the advisory committee that the pathway was not being considered, using data from multiple clinical development programs that were not available at the time of the meeting. The FDA’s reversal sparked some of the outrage over the decision. (Also see "US FDA’s Post-Aduhelm Reforms Include Updated Alzheimer’s Development Guidance, Record-Keeping On Sponsor Meetings" - Pink Sheet, 2 Jan, 2023.)
Higher Agreement Rate For Priority Than Standard Review Applications
Across new molecular entities and non-NMEs, the rates of agreement between the FDA and advisory committees were similar (84% v 87%, respectively). The agency did not follow committee recommendations as often for new drug applications and biologics license applications, compared to supplements. Those agreement rates were 84% v 93%, respectively. (See charts at the end of the story.)
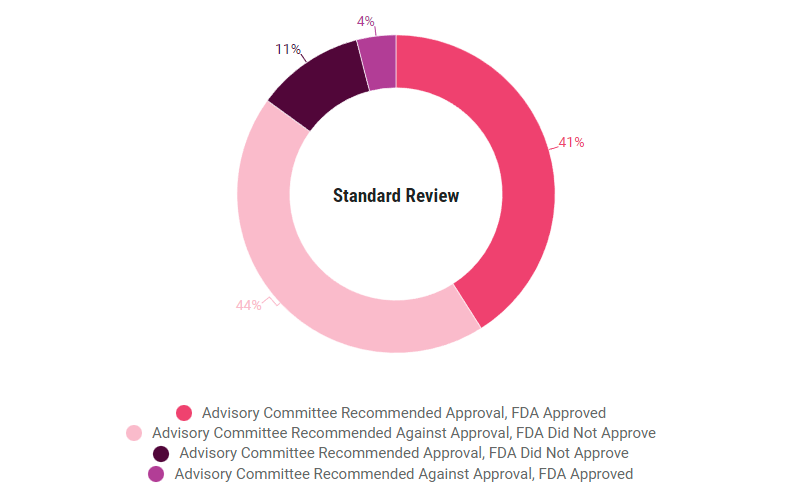
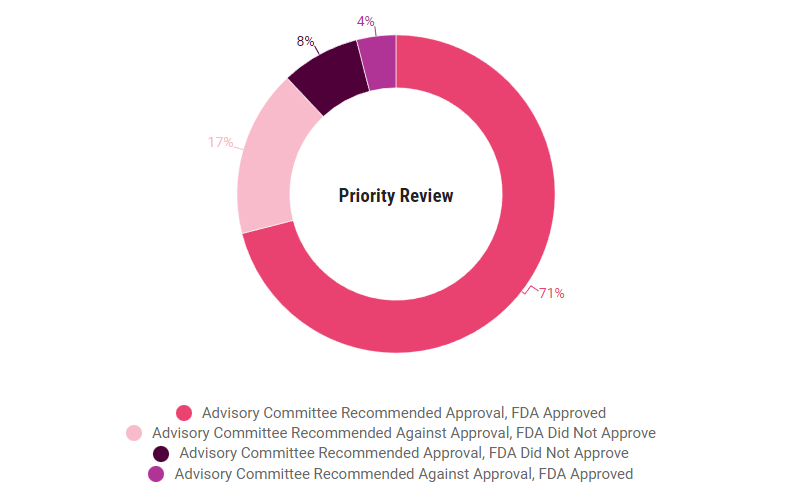
More applications used the standard review pathway than a priority review pathway, but the FDA agreed with the committee less often with standard applications, 85%, compared to priority applications, 88%. In both categories, the FDA approved 4% of the applications despite negative advisory committee recommendations.
In 71% of priority review applications, the advisory committee gave a positive recommendation and the FDA approved, compared to 41% of standard applications. Only 17% of priority applications saw negative advisory committee recommendations and a complete response letter from the FDA, compared to 44% of standard applications.
The FDA agreed with committee recommendations for orphan drugs at nearly the same rate as non-orphans, 86% v 85%, respectively, but the agreement rate to approve was higher for orphans, 59% versus 49% for non-orphans. The FDA also agreed with the advisory committee to not approve more often for non-orphans, 36%, compared to orphans, 27%.
Overall, the FDA-advisory committee disagreement rates were similar for orphans and non-orphans, 13% and 14%, respectively. However, the agency did not follow a committee recommendation for approval 11% of the time for orphans, compared to 9% for non-orphans, and approved 2% of the orphan products considered despite a negative advisory committee recommendation, compared to 5% for non-orphans.
Like the priority review pathway, the unmet needs usually addressed by orphan products tends to increase the likelihood of approval.
The fiscal year 2023 appropriations package directed the FDA to try to include at least two experts on advisory committees when they are considering orphan products. (Also see "US FDA Neurology Drugs Program Gains Boost In FY 2023 Funding Bill Amid Calls To Clarify Development Policies" - Pink Sheet, 3 Jan, 2023.) Advocates have complained that advisory committees rarely consider orphan products and members often are not familiar with their sometimes unique issues. (Also see "Senate Budget Draft Would Be Less Generous To US FDA Than House For FY 2023" - Pink Sheet, 1 Aug, 2022.)
Data Does Not Affect Meeting Strategy Much
DiBiasi said his company does not use the data on FDA-advisory committee agreement to develop sponsor strategies for the meetings. He said staff look at the issues that caused individual members to cast negative votes in the past and will try to proactively address potentially similar issues.
“It’s not outcomes as much as it is the behaviors, why people voted a certain way,” he said.
DiBiasi said the ultimate goal is to facilitate a robust scientific discussion about the product.
“It’s always best to address audience concerns no matter what kind of meeting it is,” he said.
3D Communications surveyed advisory committee members in 2021 after the Aduhelm decision and found many believed their votes should carry more influence over FDA decisions. A majority said there should be a threshold for a negative vote to block product approval. (Also see "Power To The (Adcomm) People: Members Believe Votes Should More Directly Affect US FDA Decisions" - Pink Sheet, 17 Aug, 2021.)
Application Type, Review Pathway Affect FDA, Advisory Committee Agreement Rates
NDA, BLA and supplement concordence rates are similar, but priority review agreement rates are higher than standard review rates, according to a study of 2017-2022 application decisions.
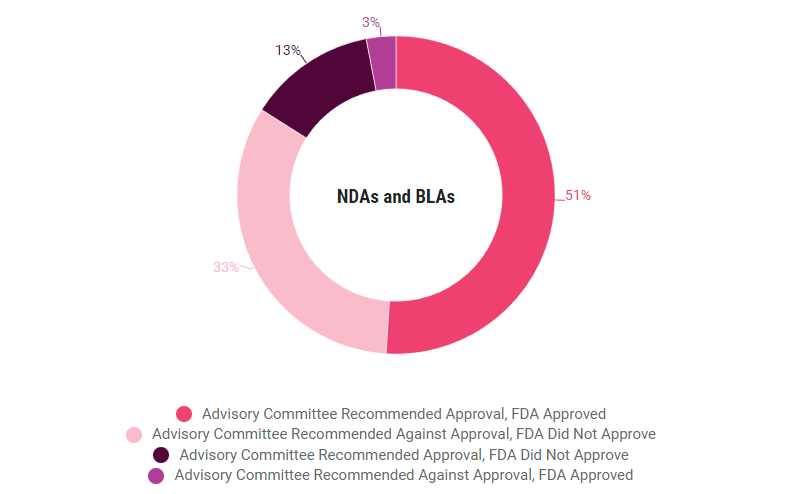
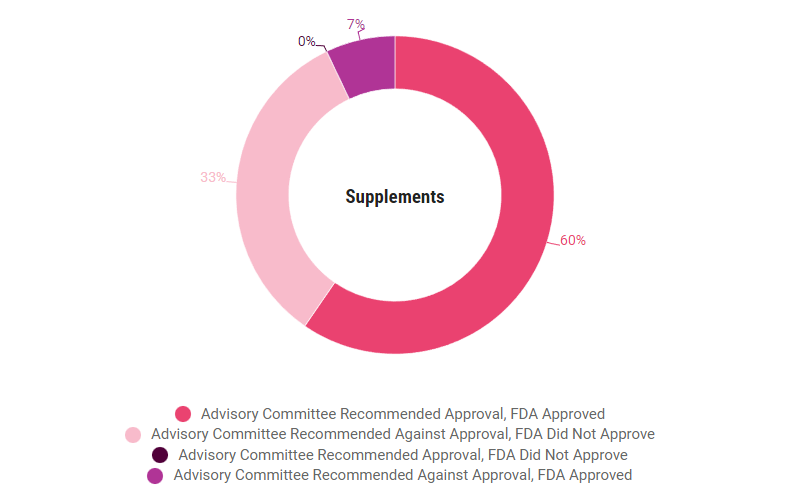
Concordence By Review Pathway
Brenda Sandburg contributed to this report.
Click here to read this article on Pink Sheet.